Early Kidney Cancer – Robotic Surgery
To better understand your symptoms, visit us for a comprehensive diagnosis and personalised treatment plan
Early Kidney Cancer – Robotic Surgery
What is kidney cancer?
The kidneys serve an important role in removing waste products from the body. Cancer of the kidney refers to the uncontrolled growth of abnormal cells within the kidney. There are many types of cancer that can develop in the kidney, the most common being renal clear cell carcinoma (RCC). It affects more men than women, and accounts for three per cent of all new cancers worldwide. Singapore has seen a two-fold increase in incidences over the last 30 years. It occurs more frequently in Chinese than Malays and Indians, and commonly occurs from age of 50 years onwards.
The following groups of patients are at increased risk of developing kidney cancer: (1) tobacco smokers; (2) obese patients; (3) patients with end-stage renal disease on dialysis; and (4) patients who develop acquired renal cystic disease (abnormal pouches containing fluid which progressively takes over the normal kidney tissue, resulting in kidney failure).
Just like other cancers, renal cell cancer starts out with a small growth in the kidney tissue, which increases in size as time passes. It usually grows as a single mass within the kidney. When this occurs, there may be more than one tumour found in a kidney or both at the same time. While some renal cell cancers are detected only after they have grown quite big, two- third of all kidney cancers are usually diagnosed before it spreads (metastasize) to the other organs such as the lung and the bony skeleton, through the bloodstream or the lymph vessels.
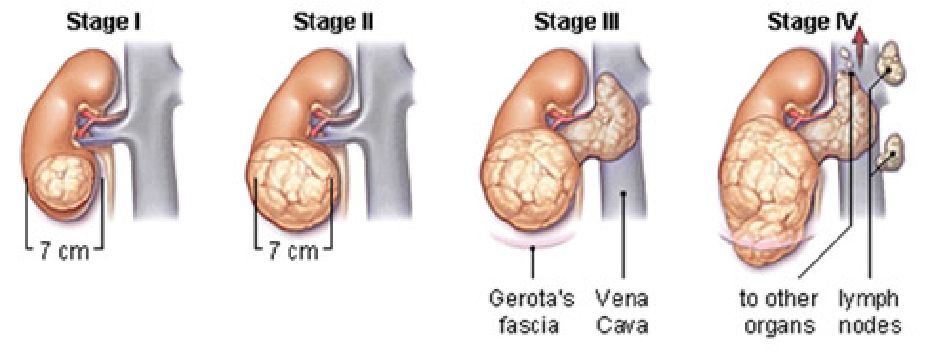
Figure 1: Stages of kidney cancer progression
Early stage 1 renal cell cancers, defined as less 7cm in size, are usually diagnosed incidentally, when patients undergo routine ultrasound or CT scans for health screening or other unrelated symptoms. In its early stages, kidney cancer usually does not cause any symptoms. As it progresses, it may cause visible blood in the urine, back pain, and weight loss. The likelihood of cancerous change is made based on the presence of features on the CT or MRI scan of the kidneys. Biopsy of such kidney tumours is no longer favoured, due to the risk of bleeding and possibility of false negative reports. 80% of such tumours found on CT scan, when removed and examined under the microscope, will turn out to be cancerous. In 20% of patients, microscopic analysis will confirm that such tumours are actually benign, and do not need further follow-up2.
Surgical excision of Stage 1 kidney tumours, whilst sparing the rest of the unaffected kidney, has now been established as the current international standard of care advocated by the European Association of Urology and American Urological Association2,3. Such nephron-sparing surgery (NSS) is now preferred to radical nephrectomy, where the entire kidney is removed. Several large-scale studies have found that patients who undergo nephron-sparing surgery, live longer and have a significantly lower risk of developing hypertension, ischaemic heart disease or strokes over the long term compared to patients who had their entire kidney removed4,5. In patients who have only one functioning kidney which does develop early kidney cancer, NSS is the only surgical approach that gives such patients the possibility of avoiding renal failure and need for lifelong dialysis6.
Nephron-sparing surgery involves five main steps: (1) identify the tumour in the affected kidney; (2) clamp the blood vessels supplying the kidney to minimize bleeding; (3) remove the tumour with a 1cm rim of healthy tissue around it to avoid leaving cancer cells behind; (4) closing the defect left in the kidney tissue, and (5) removing the clamps off the blood vessels and checking for bleeding before closing up. (Fig 2).
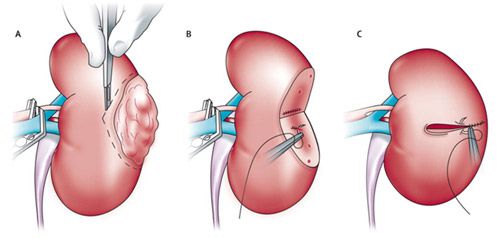
Figure 2: Steps of nephron-sparing surgery for early kidney cancer
NSS may be performed through three approaches: (1) traditional open surgery, which involves a 15-20cm incision in the abdomen or flank; (2) laparoscopic surgery, which is performed through small incisions in the abdomen; or (3) robotic surgery, using the da Vinci® surgical robot to remove the tumour and sew up the resulting defect in the affected kidney. Minimally invasive surgery, with or without robotic instrumentation, offers many advantages over traditional open surgery – small incisions result in significantly less pain, much quicker recovery, shorter hospital stays and earlier return to daily activities. For open surgery, many patients complain of chronic pain or numbness over the large incision (Fig 3).
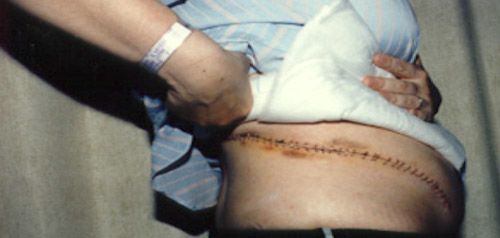
Fig 3(a): Patient with conventional open kidney-sparing surgery scar
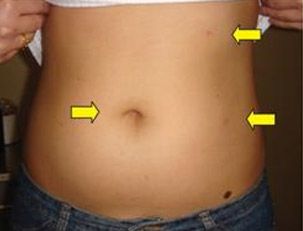
Fig 3(b): Patient with minimally invasive kidney-sparing surgery scar.
Nephron-sparing surgery is a technically more difficult operation than simply removing the entire kidney. As it involves removing the tumour from the kidney, there may be bleeding from the raw surfaces of the remaining kidney defect. There is also a possibility of a urine leak from the site of defect, if the kidney reconstruction is not performed well, which may result in postoperative infection and prolonged hospital stays. Thankfully, such complications are not common, and the large majority of patients have an uneventful recovery.
Yes, NSS carries with it a potential risk of positive margins, where cancer cells are potentially left behind. In most published series, this has been reported at less than 2%, and usually does not occur if the tumour capsule has not been breached during the surgery.
With surgeons worldwide increasingly adopting robotic nephron-sparing surgery as their preferred mode of treatment, many centres have reported impressive outcomes for robotic NSS in dealing with such challenging cancers8,9. For surgeons experienced in robotic partial nephrectomy, many of such cancers previously deemed as too difficult for NSS to be performed safely, are no longer daunting as the repertoire of surgical techniques for handling such complex tumours continue to evolve. You should explore all available options by consulting surgeons with considerable expertise in dealing with such challenging cases.
The introduction of the da Vinci® robot has empowered surgeons with many advantages over standard laparoscopic approach. Surgeons can visualize the anatomy of the kidney and surrounding structures in great 3-dimensional clarity, with optical magnification of structures up to 12 times. The range of movement of the robotic instruments allows the surgeon enhanced dexterity, improving the speed at which the tumour can be excised and the defect sewn up (Fig 4).
Fig 4: Repertoire of da Vinci® robotic instruments and their range of movement compared with the human wrist
In minimally invasive NSS, the surgical challenge lies in minimizing the clamp time on the vessels supplying the affected kidney. The longer the clamp time needed for excising the tumour and closing up the defect, the longer the kidney nephrons are starved of oxygen (known as warm ischaemia). Warm ischaemic clamp times of more than 30 minutes have been found to be associated with irreversible loss of kidney function. In this aspect, the enhanced dexterity surgeons have using the da Vinci® robot to achieve the same goals of tumour excision and kidney reconstruction, significantly reduces the clamp time on the kidney vessels and the incidence of postoperative complications, compared with surgeons using conventional laparoscopic instruments. Several published studies now validate the superior results achieved with the robotic approach.
The da Vinci surgical robot is a powerful tool for surgeons already experienced in minimally invasive nephron-sparing surgery, to reduce bleeding and warm ischaemia times. However, surgeons who have never been trained on the da Vinci® robot, will invariably require a learning curve to familiarize themselves with the manoeuvres achieved with the robotic instruments. Given the time-sensitive nature of nephron-sparing surgery and risk of irreversible kidney damage caused by prolonged warm ischaemia clamp times, it would be better for such surgeons to familiarize themselves first with technically easier surgeries such as robotic prostatectomy for prostate cancer, or robotic pyeloplasty for kidney pelvic-ureteric junction obstruction (PUJO), before attempting robotic NSS.
Stage 1 kidney cancers carry a very good prognosis if they are removed early without having positive margins. In such patients, there is an 85-90% chance that at ten years after surgery, there will be no cancer recurrence. In healthy patients who have undergone successful nephron-sparing surgery of the affected kidney, their long-term quality of health is almost comparable to a patient of similar age with two healthy kidneys.
Yes. In patients with significant co-morbidities for whom the risk of undergoing surgery and anaesthesia is high, they may consider other forms of tumour ablation such as radiofrequency ablation (RFA), or cryoablation of the kidney mass. These procedures are usually performed by radiologists experienced in interventional techniques. However, their long-term outcomes are not as good as surgical excision (particularly for tumours larger than 2cm), and there is a higher risk of cancer recurrence as the tumour is ablated but not removed from the body10.
Kidney cancers are now increasingly being diagnosed at an early curable stage. These cancers carry an excellent long-term prognosis if removed early. In addition, there is a 20% chance that such suspected cancers will turn out to be benign lesions on final analysis. Kidney-sparing surgery has now become the standard of care for such early cancers. Where the surgical expertise is available, nephron-sparing surgery using the da Vinci® robot has been found to deliver the best surgical outcomes in terms of minimizing blood loss, warm ischaemia clamp times, and postoperative complications such as urine leaks. In this age of technological advances, patients should no longer have to lose their entire kidneys for such early lesions, particularly if these turn out to be benign on final analysis.
1. Singapore Cancer Registry interim annual registry report. Trends in cancer incidence in Singapore 2008-2012. National Registry of Diseases Office, Singapore.
2. Ljungdberg B, Bensalah K, Bex A et al. EAU Guidelines on renal cell carcinoma – 2013 update. European Association of Urology. http://www.uroweb.org/gls/pdf/10_Renal_Cell_Carcinoma_LR.pdf
3. Novick AC, Campbell SC, Belledegrun A et al. AUA Guideline for the management of the clinical Stage 1 renal mass. American Urological Association 2010. http://www.auanet.org/education/guidelines/renal-mass.cfm#130
4. Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al: Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7: 735.
5. Go AS, Chertow GM, Fan D, and McCulloch CE: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Eng J Med 2004; 351: 1296.
6. Hillyer SP, Bhayani SB, Allaf ME et al. Robotic partial nephrectomy for solitary kidney: a multi-institutional analysis. Urology 2013;81(1):93-97.
7. Sprenkle PC, Power N, Ghoneim T et al. Comparison of open and minimally invasive partial nephrectomy for renal tumours 4-7cm. Eur Urol 2012; 61(3): 593-599.
8. Long JA, Yakoubi R, Lee B et al. Robotic versus laparoscopic partial nephrectomy for complex tumors: comparison of perioperative outcomes. European Urology 2012; 61(6): 1257-1262.
9. Borghesi M, Schiavina R, Gan M et al. Expanding utilization of robotic partial nephrectomy for clinical T1b and complex T1a renal masses. World Journal of Urology 2013; 31(3): 499-504.
10. Klatte T, Shariat SF, Remzi F. Systematic review and meta-analysis of perioperative and oncological outcomes of laparoscopic cryoablation versus laparoscopic partial nephrectomy for the treatment of small renal tumors. J Urol. 2013 Nov 11. doi:pii: S0022-5347(13)05900-4. 10.1016/j.juro.2013.11.006.
